- (In)Fertility Journey- 9. IVF Considerations
- (In)Fertility Journey- 8. IVF After Egg Retrieval
- (In)Fertility Journey- 7. IVF Egg Retrieval
- (In)Fertility Journey- 6. IVF Stimulation
- (In)Fertility Journey- 5. Decisions regarding IVF
- (In)Fertility Journey- 4. Preparing for IVF
- (In)Fertility Journey- 3. Before IVF- Hope
- (In)fertility Journey- 2. Background
- In(Fertility) Journey- 1. Introduction

Decisions: Important!
For us, making the decision to do IVF was a huge decision that was a lot already. I won’t go into that, it’ll be different for everyone and very personal.
There are some major decisions to make within the decision to do IVF. And we weren’t really aware of this, but it would have been helpful to have made these decisions going into our first consult. Because that was the first and really, last time we consulted with the doctor in person during that cycle. The doctor set the course of our treatment after that first initial consult. After that, any questions, concerns, directions different from his initial recommended course was a change to the existing plan, and required additional effort on our part to communicate.
I’ll go over some decisions that in hindsight I wish we had gone over. If we had gone over some of these in the initial consult, it would have made the whole thing easier, because each little thing in this process starts to build on itself- there are so many details. Or maybe you’re the type of person for whom all this would be too overwhelming, in that case, feel free to skip this post.
Before I go into all the decisions, it’s important to remember that this process is an issue of numbers. The goal is to maximize the chances of a healthy baby, balanced by the goal of minimizing negative impacts on the mother, egg, embryo, pregnancy, etc. Also, to minimize the negative impacts on the father and everyone else involved. Every step of the process involves attrition. To this end, I found this summary of the numbers quite helpful to keep in mind. The process is highly variable depending on your health condition and age. This is taken from fertilityiq.com.
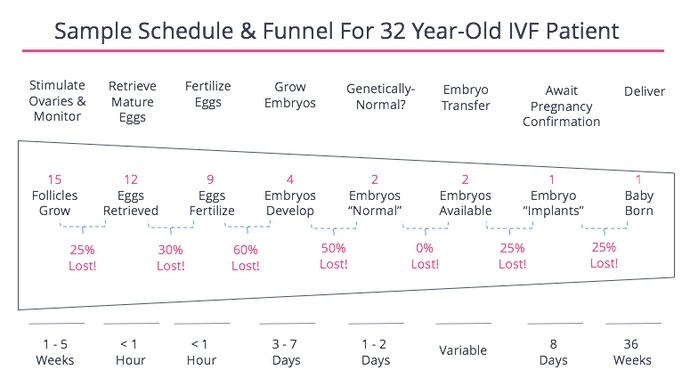
There’s a lot in this process that is beyond your control, other than picking a clinic and a doctor. The following are some items that you could have some control over, that could impact the outcome of your own IVF schedule/timeline.
Choosing an IVF Clinic & Doctor
In hindsight, I think this is one of the most important decisions going into IVF. I’ve mentioned before, but IVF clinics in the U.S. are not clearly regulated (as it seems to be in Europe). Clinics may voluntarily report to the Society for Assisted Reproductive Technology, or Sart.org. The CDC also maintains a website of Assisted Reproductive Technology success rates, but like Sart, it is only reporting clinics that are members of Sart or used an accredited embryo laboratory. Sart member clinics are subject to the requirements copied below (from https://www.sart.org/professionals-and-providers/join-sart/)
- Annual submission of cycle-specific clinic outcome data to the SART Registry, verified by Medical Director, with permission to disclose such data to the public and to allow data to be validated. Approximately 8-10% of reporting clinics are validated each year.
- Accreditation of the Embryology Laboratory every two years by CAP or Joint Commission.
- The Medical Director of any new practice must be REI subspecialty certified by ABOG, an active candidate for subspecialty certification, or grandfathered in by having been Medical Director of a SART member practice prior to 1/1/00.
- Effective January 1, 2006, all ART Laboratory Directors must meet uniform criteria. Physicians not currently Lab Directors must complete the RE fellowship training in the Lab Director track, attain sufficient hands-on experience to meet ASRM/SART guidelines and pass a certification exam administered by ABB. Nondoctoral lab directors and physicians serving as Lab Directors prior to l/l/06 may continue to serve in this capacity. Nondoctoral Lab Directors need to have been serving as director prior to July 20,1999.
- Adherence to all ASRM/SART Guidelines including ethical, practice, advertising, and laboratory.
- Fulfillment of all financial obligations regarding dues and fees.
- Continued provision of IVF services. Active members must perform a minimum of 20 follicular aspirations and/or transfers annually.
Some things to keep in mind; the numbers reported by a clinic are self-reported. It could be the critical-thinking scientist in me, but this point must not be overlooked. There is likely to be immense pressure to report positive numbers. When looking at the Sart.org reports, make sure you understand how to read the report by checking out their tutorial: https://www.sart.org/patients/fyi-videos/understanding-the-sart-clinic-report/.
Notice also, that while there are certain requirements of the medical and laboratory director, there is no mention of requirements for laboratory staff. Chances are the great majority of the work would be done by laboratory staff, and how technically skilled and careful they are can vary widely.
Another thing to keep in mind is that clinics are mostly self-regulated; while some of the procedures are standardized to industry standards due to ease and availability of materials, many of the methods employed are specific to each clinic. That is because clinics are not regulated and required to adhere to the same methods/procedures. As an example, there are currently over 20 different commercially available IVF culture media used for embryo culture (For an article examining IVF culture media, see here). The culture media has critical impacts on the outcome.
In addition to the possible different culture media, there are many possible differences in stimulation protocol, retrieval protocol, culture conditions, etc. This is why the ‘success’ rate of one clinic may differ tenfold from another. There are actual real differences in the outcomes. A ‘good’ clinic, a ‘good’ doctor, can improve your chances of success in a very real way, and it’s worth your time to try to find the best one.
We did not know anyone in our area undergoing IVF, so we couldn’t ask friends for their recommendation/experiences. We relied on the Sart.org reports and reading reviews on Yelp. One resource we did not use, because we did not know about it until after we had already started on IVF, was Resolve.org. Because we haven’t used this, I can’t speak to how good this website is, but the purpose of this organization is to put you in touch with locals who are also undergoing fertility treatment. I wish I had joined this group much earlier and asked for their recommendation on clinics/doctors.
Finally, if you know someone who has undergone IVF, see if they are willing to share their experience(s) with you. This could really help you in identifying a clinic and/or doctor by giving you some examples of what worked/didn’t work for them.
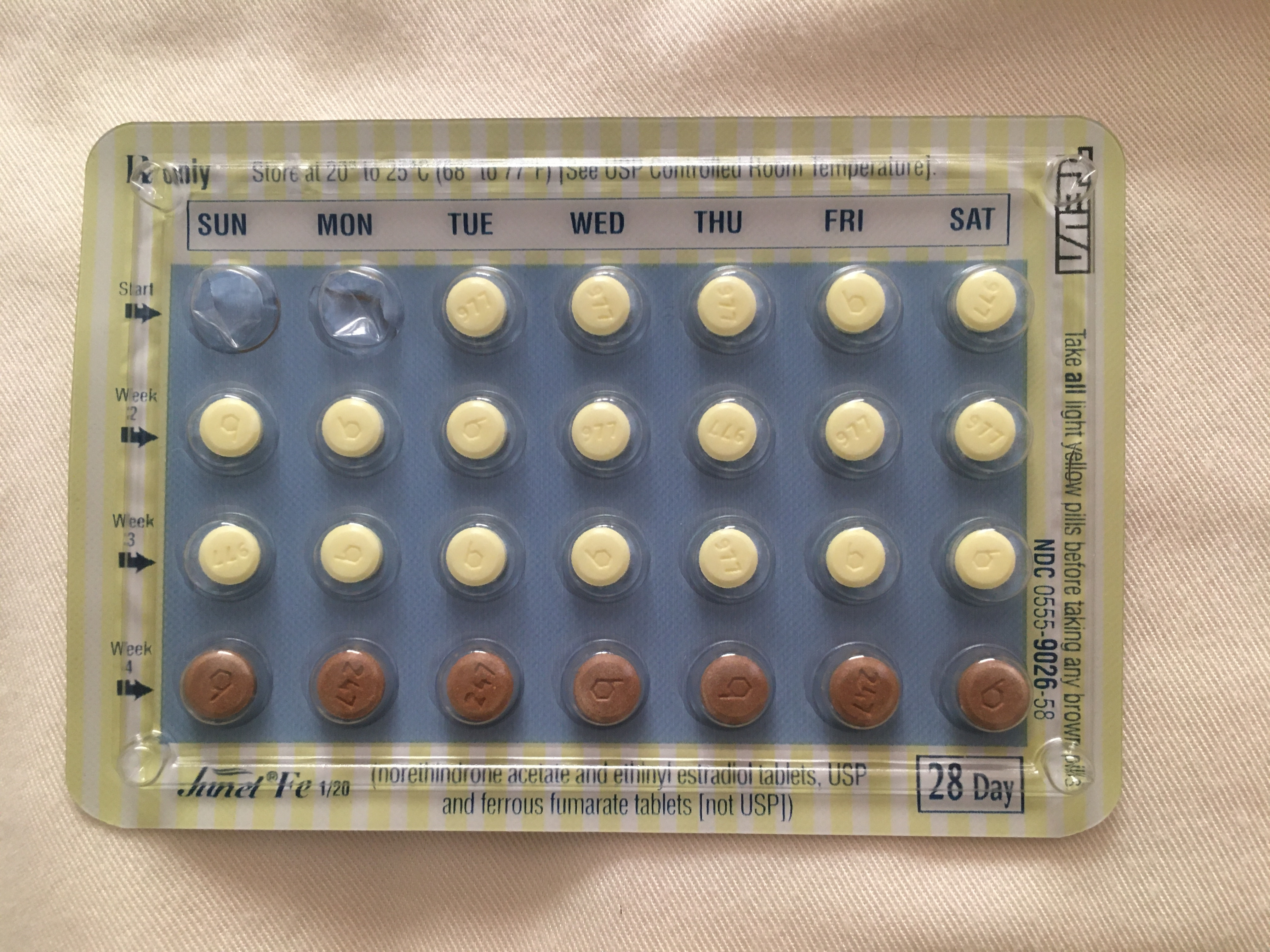
Birth control length during Prep Month.
I would have liked to have taken no more than 1 packet of pills at most. Because I was prescribed 22 active pills and there was only 21 in each packet, and I could only get one packet at a time, I had to go to the pharmacy twice instead of just once. It was a pain during COVID. Also, if you’re a person that is very sensitive to birth control, this is something that seems to be quite flexible, and I would definitely discuss this with my doctor next time.
How this impacts the timeline: Who knows? It basically determines the starting point of this entire IVF cycle.
Stimulation Plan.
This particular item we did touch on during our initial consult. All my labs look fine and I knew I was sensitive to drugs, so we wanted to move forward with a moderate stimulation. I was really afraid of high doses of stimulation and what that might do to me in general. We ended up with a plan of 225 IU of Gonal-f and 75 IU of Menopur daily, for a combined total of 300 IUs of FSH (and some LH) stimulation.
According to a number of sources, 150 IUs is low stimulation and often gives poor results while over 450 IUs might be considered a higher stimulation. The protocol really varies a lot across the board. Fertilityiq.com states that most IVF patients receive 250-450 IUs per day.
The stimulation plan is important to maximize the number of eggs that can be retrieved while balancing the danger of Ovarian HyperStimulation Syndrome (OHSS more here), and possibly, negative effects of hormonal balance if a fresh transfer is planned. The greater the stimulation, the greater the risks of OHSS. The information on fertilityiq.com also goes over the data that suggests more eggs retrieved is not always better for the final outcome, the live birth rate (see here). So all the more reason to use the lowest possible effective dose.
Despite however much you do research on this topic, it’s a lot to absorb. There are a lot of drugs out there used for fertility, and many of them are off-label use. So unless you know the drugs planned, it’s very difficult to do research on it. The problem is that for us, we didn’t get the stimulation plan, with the name of the drugs and the dosages until after our first appointment. We asked the nurse assigned to us many questions, but she really wasn’t in the position to clarify why the particular drugs were chosen. I would definitely ask my doctor what the plan and the drugs are, and why, next time.
Fresh or Frozen
When we were presented with this choice, of either implanting a fresh embryo, versus freezing the embryo and then implanting after a month of rest (Frozen Embryo Transfer, or FET), we were presented with data that show frozen was far more likely to result in a pregnancy, as the uterus had time to recover from stimulation and could be primed specifically for embryo implantation. All the data seems to support this. This is, of course, a huge benefit.
There were additional benefits for FET:
An FET with a month of rest in between egg retrieval and embryo implantation minimized the stress on the woman’s body, and greatly reduces the severity of possible OHSS. Because embryos for FET are typically cultured to the blastocyst stage, this lends itself to Preimplantation Genetics Testing (PGT), which currently removes a few cells from the blastocyst stage embryo. Another advantage with FET is it allows scheduling of embryo transfer at a later, flexible time. So much of IVF is so sensitive to timing, that this additional flexibility is a very real advantage for not only the fertility clinic, but also to the couple undergoing this process. We didn’t really consider the downsides.
What we did not consider is the difference in length of culture for fresh transfers vs. frozen transfers. Fresh transfers can take place after about 2-6 days in culture. Frozen transfers involve freezing the embryos after developing about 2-6 days in culture, but some clinics tend to only freeze embryos that have developed to a full blastocyst (5-6 days in culture). This was the case for our clinic.
For one, FET requires culture of embryos for 5 days (in our case). In a fresh transfer, an embryo can be transferred after only 3 days of culture. This minimizes the number of days an embryo has to survive and continue to develop in the artificial environment of the IVF lab.
This longer incubation in vitro could lead to requiring more ‘assistance’, such as ‘assisted hatching’ where an incision is made to help the embryo break free of the egg shell. The risk of embryo damage with assisted hatching is unclear.
Another thing to consider, the embryo might not survive the freezing or thawing process necessary for a FET. These are just more hurdles that an embryo has to jump through, and depends greatly not only on the quality of the egg and sperm, but the techniques and protocols of the embryology lab associated with the fertility clinic.
Standard Fertilization or IntraCytoplasmic Sperm Injection (ICSI)
In ICSI, the embryologists look for the ‘best’ sperm based on motility and shape, and manually inject this sperm into the egg. This is typically recommended where there is concern regarding sperm quality. So in cases where one of the metrics of the semen analysis is below average, then ICSI is recommended.
However, the regular semen analysis is incomplete; a sperm that looks alright on the cellular level might not be competent to get through the layer that surrounds the egg to penetrate and fertilize. A new test available at some clinics called the Cap-Score Sperm Function Test is taking a step in that direction. We did not know about this test going into our IVF cycle.
All we knew going into our IVF cycle was that ICSI was ‘recommended due to maternal age’. Which didn’t make any sense to us at all. Additional research indicates that while ICSI is a great tool for couple with sperm issues, that there are certain increased risks of miscarriage and sex chromosome abnormalities associated with ICSI (see here).
The benefit of ICSI is that you may get an increased number of ‘fertilized’ eggs (of the eggs that are mature, as only mature eggs are injected), but the downside was there is increased risk of damage to the egg during the procedure in addition to the risks mentioned above.
Some clinics are willing to fertilize some eggs via the traditional method and some eggs via ICSI, a ‘split ICSI’. Our clinic was willing to do this, but as we tried to find more information on this, we found that our clinic was planning to do a split treatment only if a certain number of eggs were retrieved; below this number they would treat all the eggs to ICSI. Because of all this, we decided to go with traditional fertilization.
In hindsight, I wish I had asked about more possible sperm tests available before starting IVF; it’s so much work and time and money to get eggs, sperm is so much easier to test and is 50% responsible for the outcome, why not figure out more about it before diving into IVF?
Preimplantation Genetics Testing (PGT) or not?
Currently there are several types of PGT; the most common are type A, which looks at the number of chromosomes, and type M, which looks at specific gene abnormalities. For couples who are known carriers of a specific genetic condition, type M makes sense. For couples who are older, type A makes sense (this would detect conditions that are more common in older couples, such as Downs Syndrome).
The benefit of PGT is to decrease the probability of an embryo that has a defect to avoid implanting an abnormal embryo and facing failure to implant, spontaneous or stimulated miscarriages, or possibility of an infant with genetic abnormalities. Note that I say ‘decrease the probability‘ because PGT cannot capture all possible genetic defects. This is part of the reason why you sign so many consents for PGT testing.
There are several reasons for this. One, only a small subset of cells are sampled to represent the whole embryo, and those cells might not be a perfect representative of the cells that develop into the embryo, and subsequently, into the infant. Currently the practice is to remove some of the cells in the embryo that forms the placenta for PGT analysis (the trophectoderm). This avoids taking cells from the area of the embryo that develops into the infant to minimize damage to the fragile inner cell mass. The problem with this is that while it seems the trophectoderm cells are reasonably similar to the inner cell mass, it’s not 100% necessarily the same. Of the approximately 100-200 cells that make up the blastocyst embryo, not all the cells are necessarily genetically identical. As the embryo grows via cell division, each division event can introduce defects. Thus an embryo is likely to be a collection of genetically similar cells, not genetically identical cells. The result of this test relies on the small number of cells (3-10 cells) representing the whole, and this may not be the case. You may capture cells that are genetically defective, but the cells that make up the embryo are genetically normal.
As only 3-10 cells from the overall 100-200 cells are tested, there is also a chance that genetically defective cells are not captured. So there is a possibility that a PGT comes back normal, but the resulting embryo has genetic defects. Here’s a blog of a couple struggling with Spinal Muscular Atrophy.
Another reason PGT results are complicated is that it is possible that the biopsy captures cells that are genetically ‘normal’ along with cells that are genetically ‘abnormal’; this is call ‘Mosiacism’. There’s not a whole lot of understanding regarding these situations. It is unclear if having genetically different cells is part of the normal course of embryo development. There are suggestions that cells in the inner cell mass, which form the infant, is under different pressures than the cells in the trophectoderm that form the placenta. There is a possibility that the cells that form the infant is under greater pressure to select for genetically ‘normal’ cells, that an embryo that tests as abnormal in the trophectoderm, or mosiac, can self correct, and develop a genetically normal fetus. What that possibility is, is unclear, simply not enough is known about it.
The negatives associated with PGT, other than the complications in interpreting the results, is that it involves removing a few cells from a delicate embryo. This can cause irreparable damage. In addition to removing the cells for PGT, the process of removing the cells could include additional measures, such as assisted hatching (cutting an opening on the egg shell), which all have the potential of causing critical damage to the embryo.
Lastly, the current practice is to remove cells for PGT from a 5 Day old blastocyst, instead of a Day 3 embryo. Fewer embryos make it from Day 3 to Day 5. There are suggestions that Day 5 blastocysts are the gold standard; implantation of Day 5 blastocysts have a higher success rate for implantation and subsequent live birth, but these studies are difficult to conduct. It seems to select for more robust embryos, which decreases the number of embryos to work with. Keep in mind that ‘robust embryos’ in this context refers simply to embryos that survive the technique, culture conditions, and media utilized by the clinic. The PGT results for these embryos could still come back ‘genetically abnormal’.
These are all things to weigh in deciding whether to opt for PGT. Because each clinic is different, if planning PGT, you might want to ask what other add-on procedures will be taken for PGT analysis. Something to keep in mind; when deciding to do PGT, there might be greater pressure to also do ICSI; because ICSI selects a single sperm for fertilization, it can minimize complications for PGT analysis.
Finally, if you don’t decide to do PGT, genetic testings can (and often are standard procedures) be done after pregnancy is confirmed at 10 weeks via blood samples from the mother. These Non-Invasive Prenatal Testing (NIPT) screenings are much less traumatic on the developing embryo/fetus. These genetic tests can cover the same conditions that are tested with PGT. Arguably, these tests can be more accurately representative of the genetic status of your infant, as the fetus is now developing beyond just a mass of cells. I won’t go too much into this, as I don’t have personal experience with these tests.
Optional Add-ons
These would be different for each clinic. The first clinic we approached never mentioned endometriosis/endometrisis testing. The second clinic (where we went ahead with IVF) suggested the ReceptivaDx test that indicated I might have endometritis. It never occured to me to ask, and I wish I did ask about the possible add-ons. If I did, I might have decided that I didn’t want to do some of the stuff or add on other things. Someone else might want to do some of these add-ons if they knew about it. It’s nice to think the doctor would think of all the possible necessary/beneficial add-ons based on your history, but the doctor is human and likely has lots of patients, and you know your body better than anyone else. This is also a great opportunity to ask why your doctor would go with a particular add-on. A resource to look at the various possible add-ons by the Human fertilisation & Embryology Authority in UK can be found here.

Rant: Choosing IVF despite the lacks in: Information, data collection, data sharing, etc.
IVF seems to be a common word that most adults think they comprehend. As we went through the process, I found more and more resources that showed me how little we know about it. This is even with a scientific background; there were so many things I did not know.
I think one of the biggest questions is whether IVF is something a person would want to do. One can find a fair amount of information online, from women who’ve gone through the process, from clinic webpages, from fertility organization websites, and even a good number of scientific articles via the National Library of Medicine, National Center for Biotechnology Information (or PubMed.gov).
I’ll admit that while I did some research into IVF, it was when we had already begun the process that I truly did my research. I was shocked by how little the field was regulated, how vastly different the administration of care of IVF was for each clinic, and the absence of tracking information.
These are concerns, even if IVF ‘works’. After all, the goal is to grow one’s family, and then to have time to spend with them. If the IVF treatment negatively impacted the woman’s health, the relationship between the partners, the health of the children born, the financial stability of the household- are these acceptable risks? I understand these concerns also apply to people who get naturally pregnant, but IVF is an elective procedure that requires time, financial resources (for most people in California), effort, hope, support and physical/mental resilience before even getting pregnant. The result might be a happy one, but there’s also a good chance of a poor result despite everything that went into it.
Here is an article written by someone for whom IVF actually worked, on the first try, but would not recommend IVF. I’m not trying to scare people from IVF, I just think that knowing more about IVF and the unknowns would really help people make decisions they can be at peace with.
Another website I would recommend is the Human Fertilisation & Embryology Authority (hfea.gov.uk) in the UK, where there are more regulations compared to the US. It’s a good source of information. I wish there was something like this in the US. One thing that this website has, which I think is great, is rating for clinics; this include an Inspection rating, as well as patient ratings.
A website that explores the darker side of IVF, is Reprotechtruths.org, the “The untold stories of IVF”. I think these perspectives are also helpful if someone decides not to do IVF or decides to stop further IVF treatment.
The increase chance of pregnancy with multiple IVF cycles. It can be really hard to undergo IVF, have a poor result, learn more about how much one did not know about IVF, and feel the constant urge to ‘address’ or ‘correct’ the protocols in hopes of a better outcome. There can also be the idea of ‘sunk costs’, that it hurts to have put so much (time, hope, money, etc.) into a cycle that did not have the outcome hoped for.
I understand that there can be a certain amount of adjustments, as each person is different, and a failed cycle can provide useful information. But what really bothers me is that there is no formal way for clinics to share this information in the US, and moreover, that it is not in the economic interests of clinics to do so.
Meanwhile, repeating IVF cycles as a result of failed cycles provides more business for clinics. I’m not saying that my clinic, or any clinic would deliberately not do everything within their power to ensure a good outcome on the first IVF cycle, I’m simply stating that the numbers work out that clinics get more business and make more money with each additional cycle.
There seems to be poor incentive to spend a lot of time and effort for a doctor in these clinics to analyze a patient’s health history and run every possible relevant test and assess those results before crafting a customized IVF plan. It’s easier for each clinic, each doctor, to throw a standard protocol at the wall, see what sticks, and make adjustments as necessary. I’m not saying this isn’t a possible working strategy; it’s just really hard on IVF couples, and results in patchy success rates for individual IVF clinics. This could be so much better if the data from each clinic was compiled together to generate a much larger data pool that can provide more statistically significant data. In my fantasy, this searchable database would have data submitted by the clinic but also have the ability for the patients to submit their IVF data to improve accuracy.
One of my biggest struggles is what while I’m aware of the IVF plan up to egg retrieval, once the eggs are collected from my follicles, they go into the black box of the embryology lab, where I have no control on how they are handled or any information on the type or quality of work. As someone who is very familiar with sterile lab work, I can think of so many ways things can go wrong. As a person who has worked in government labs where the results were of critical importance, I am familiar with Quality Assurance plans, Quality Control Measures, Proficiency tests, and Verification by another technician for every step of the process. As someone who produced novel scientific work, I am used to the copious amount of observations made at every step of an procedure. And yet, the only person required to meet any sort of criteria in the embryology lab is the lab director. Embryology labs provide no more information other than the number of eggs/embryos that have made it to Day3 or Day5. This disturbs me greatly. All the information that might be helpful for troubleshooting an unsuccessful cycle is either not collected, or not shared.
According to the 2018 National Summary provided by the CDC, approximately 30% of cycles result in a pregnancy. So approximately 70% of cycles end in failure to result in a pregnancy. This somewhat fits the three IVF cycles being a good number to try that I’ve seen in many places.
In the UK, the IVF success rates between 2014-2016 are as follows:
- 29% for women under 35
- 23% for women aged 35 to 37
- 15% for women aged 38 to 39
- 9% for women aged 40 to 42
- 3% for women aged 43 to 44
- 2% for women aged over 44
In Canada, the IVF success rates for 2013 are as follows:
The clinical pregnancy rates per cycle started, by age of the mother, were:
- 34% for women under 35 years old
- 28% for women aged 35-39 years
- 15% for women 40 years old and over.
The singleton pregnancy rates per cycle started, by age of the mother, were:
- 27% for women under 35 years old
- 20% for women aged 35-39 years
- 11% for women 40 years old and over.
Canada has a great IVF blog, IVF.ca, where lots of good information can be found. In addition, Canada seems to have more regulations regarding IVF compared to the US. Whether this has something to do with their better rates is unclear.
All this is to say, you might be one of the lucky ~30% for whom IVF works on the first try. It’s important to also keep in mind that ~70% of couples, it doesn’t work on the first try. I think these numbers are helpful to consider before going into IVF, to plan how many cycles you would be willing to try. This might help in making some baseline decisions before you get wrapped up in the overwhelming experience of IVF, during which all kinds of drugs and hormones make everyday stuff feel hard, not to mention learning more about IVF and making big decisions.
